On June 13, 2022, medical history was made when the U.S. Food and Drug Administration (FDA) approved Olumiant™, a medication to treat alopecia areata. This exciting medical breakthrough announcement marks the first FDA-approved treatment for alopecia areata.
“This is the dawn of a new era,” Nicole Friedland, President and CEO of the National Alopecia Areata Foundation, said in an organization press release. “For the first time, alopecia areata patients have the option of an approved treatment that has undergone rigorous testing in clinical trials. We anticipate more treatments to come, bringing additional choices to our community.”
What is Alopecia Areata?
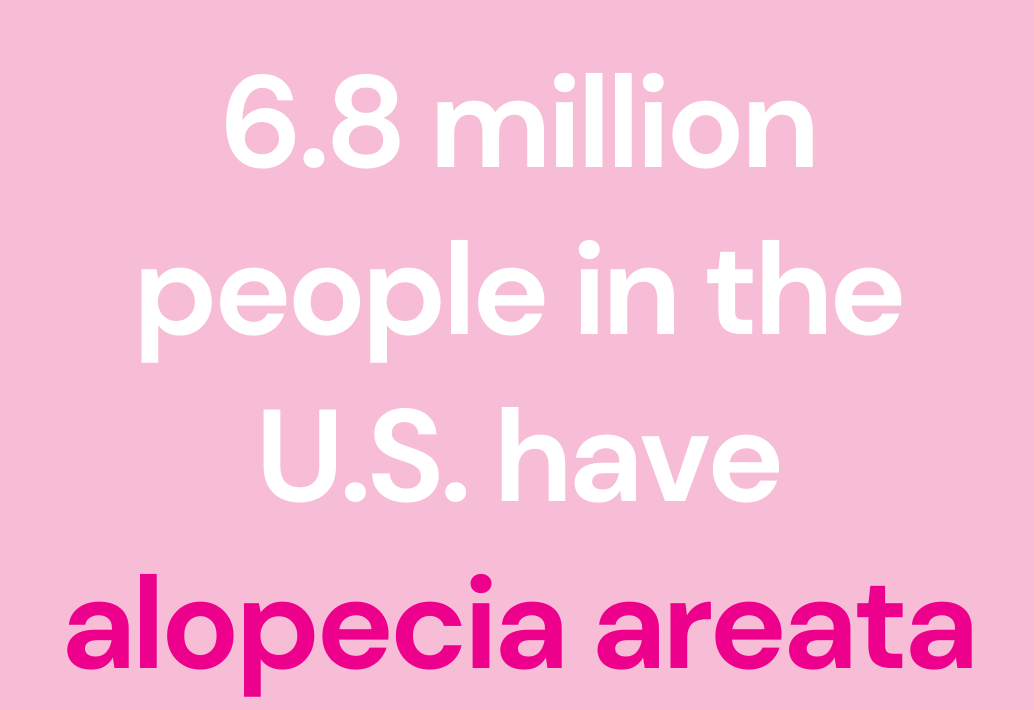
According to the National Alopecia Areata Foundation, alopecia areata is a common autoimmune disease that affects roughly seven million Americans (including more than 300,000 people with severe levels of the condition). Alopecia areata may cause either total or partial loss of scalp and body hair.
Understandably frustrating to those who live with the condition, scientists have yet to identify what “triggers” the immune system to attack the body’s healthy hair follicles. Additionally, some people with alopecia may experience psychological consequences, including high levels of anxiety and depression.
Symptoms of alopecia areata:
- Small, round/oval patches of hair loss on the scalp, beard area of the face or other areas of the body with hair
- Hair loss and regrowth at the same time in different areas of the body
- Significant hair loss in a short period of time
- Hair loss that’s mostly on one side of the scalp, rather than both sides
- “Exclamation point” hairs that are narrow at the base/next to the scalp
- “Stippling” or “pitting” (rows of tiny dents) on the fingernails
What is Olumiant?
Olumiant is a new FDA-approved oral tablet. It belongs to a class of medications known as Janus kinase (JAK) inhibitors which block the activity of one or more of a specific family of enzymes, interfering with the pathway that leads to inflammation. Olumiant is tended for adult patients with severe alopecia areata. Its availability means people with alopecia areata now have a treatment option for the entire body rather than just a specific location.
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” said Kendall Marcus, M.D., director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, via a press statement.
The efficacy and safety of Olumiant in alopecia areata was studied in two randomized, double-blind, placebo-controlled trials (Trial AA-1 and Trial AA-2) with patients who had at least 50 percent scalp hair loss (as measured by the Severity of Alopecia Tool) for more than six months. Patients in these trials received either a placebo, two milligrams of Olumiant or four milligrams of Olumiant per day. The primary measurement of efficacy for both trials was the proportion of patients who achieved at least 80 percent scalp hair coverage at week 36.
In Trial AA-1, 22 percent of the 184 patients who received two milligrams of Olumiant and 35 percent of the 281 patients who received four milligrams of Olumiant achieved adequate scalp hair coverage; compared to five percent of the 189 patients who received the placebo. In Trial AA-2, 17 percent of the 156 patients who received two milligrams of Olumiant and 32 percent of the 234 patients who received four milligrams of Olumiant achieved adequate scalp hair coverage; compared to just three percent of the 156 patients who received the placebo.
Common side effects associated with the use of Olumiant included upper respiratory tract infections, headache, acne, high cholesterol, increase of an enzyme called creatinine phosphokinase, urinary tract infection, liver enzyme elevations, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, genital yeast infections, anemia, low number of certain types of white blood cells, abdominal pain, shingles and weight increase.
Olumiant comes with other warnings, so patients should discuss their personalized care with their physician to determine if this new medication is an appropriate treatment option. Olumiant is also approved as a treatment for certain adult patients with active rheumatoid arthritis and, in certain hospitalized adults, the treatment of COVID-19.
Learn More
The National Alopecia Areata Foundation (NAAF) supports research to find a cure and/or acceptable treatments for alopecia areata, plus supports those with the disease and educates the public about alopecia areata. For more information about alopecia areata, visit the organization’s website: naaf.org.







